Abstract
Background
Dual overexpression of BCL2 and MYC in diffuse large B-cell lymphomas, termed double-expressor lymphoma (DEL), is associated with poor outcomes after treatment with chemoimmunotherapy consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP). The use of R-CHOP versus more intensified regimens remains controversial and is due to the lack of clear data to guide clinical decision-making. Retrospective studies have reported mixed results with the use of dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-EPOCH) for the treatment of DEL, but the previously largest published retrospective study suggests a clinical benefit in patients <65 years with DEL when compared to R-CHOP. To better define the role of dose-intensifying regimens in managing DEL, we conducted the largest retrospective analysis to date comparing the outcomes of R-CHOP and R-EPOCH as frontline treatment for DEL.
Methods
We retrospectively analyzed 130 adults with DEL at the University of California campuses in Davis, Irvine, and Fresno who were diagnosed from January 2005-February 2021 and received first-line treatment with R-CHOP or R-EPOCH for DEL. DEL was defined as expression of MYC ≥40% and BCL2 ≥50% by immunohistochemistry (IHC). Triple expression was defined as DEL + BCL6 ≥50% by IHC. MYC, BCL2, and BCL6 rearrangements (-R) were determined by fluorescence in situ hybridization (FISH). The primary endpoint was 2-year progression-free survival (PFS), and secondary endpoints were 2-year overall survival (OS), overall response rate (ORR), complete response (CR) rate, and duration of response (DoR). Time-to-event analyses were conducted using the Kaplan-Meier method and were compared by a log-rank test. Univariate and multivariate analyses were conducted via the Cox proportional hazards model. Patients alive at the end of the study were censored at their last follow-up date. Responses were evaluated via the Lugano criteria.
Results
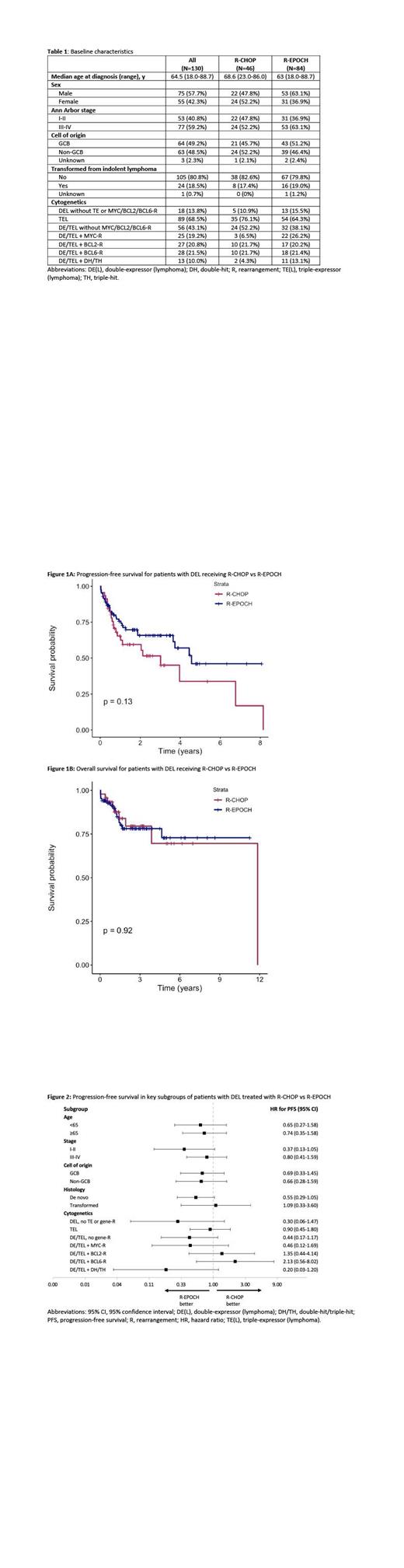
Baseline characteristics of the 130 DEL patients are listed in Table 1. Forty-six received R-CHOP, and 84 patients received R-EPOCH. The median follow-up for the R-CHOP and R-EPOCH groups were 18 months (range, 2-143) and 22 months (range, 1-136), respectively. The median number of cycles competed for each group was 6 (range, 1-8).
The 2-year PFS and OS with R-CHOP vs R-EPOCH for the overall population were 60% (95% confidence interval [CI], 46-77) vs 66% (95% CI, 56-78) (Figure 1A), and 79% (95% CI, 66-95) vs 78% (95% CI, 69-89) (Figure 1B), respectively. For patients <65 years old, the 2-year PFS for those receiving R-CHOP vs R-EPOCH were 60% vs 71% (p=0.34), and the 2-year OS were 72% vs 88% (p=0.15), respectively. The ORR and CR rate for R-CHOP were 89% and 78%, and for R-EPOCH were 85% and 75%, respectively. For patients achieving a partial response (PR) or CR, the median DoR was 2.75 years for R-CHOP, and was not reached for R-EPOCH (p = 0.0067). A univariate analysis among all 130 DEL patients of age ≥65, stage ≥3, cell of origin, transformation, triple expression, MYC-R, BCL2-R and/or BCL6-R, and double-hit/triple-hit (DH/TH) status revealed that MYC-R and DH/TH were significant for worse PFS, hazard ratios (HR) 2.10 (95% CI, 1.11-3.95) and 3.64 (95% CI, 1.79-7.42), respectively. Only DH/TH was significant for worse OS, HR 4.20 (95% CI, 1.65-10.66). None of these factors were significant in a multivariate analysis for PFS or OS. A separate subgroup univariate analysis of R-CHOP vs R-EPOCH is provided in Figure 2. In total, 14 received an autologous stem cell transplant during their treatment course for relapsed/refractory disease, 7 in the R-CHOP group, 7 in the R-EPOCH group.
Conclusion
In this multi-center study, R-CHOP and R-EPOCH led to similar OS and PFS rates in patients with DEL. However, R-EPOCH led to more durable remissions than R-CHOP for those achieving a PR or CR. Across multiple subgroups of DEL by age, stage, cell of origin, transformation, triple expression, and MYC-R, BCL2-R and/or BCL6-R, neither regimen demonstrated superior outcomes. Larger randomized, prospective trials are needed to clarify the role of dose-intensified regimens for first-line treatment of DEL.
Brem: TG Therapeutics: Consultancy; KiTE Pharma: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Morphosys/Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics/Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SeaGen: Speakers Bureau; BeiGene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees. Abdulhaq: BMS, Alexion, Oncopeptides, Morphosys, Pfizer, Norvartis: Honoraria; Oncopeptides, Alexion, Amgen: Speakers Bureau; Morphosys, BMS, Amgen: Membership on an entity's Board of Directors or advisory committees. Reid: ADC Therapeutics: Other: Serves as Principle Investigator, Research Funding; Aptose Biosciences: Other: Serves as Principle Investigator, Research Funding; Millennium Pharmaceuticals: Other: Serves as Principle Investigator, Research Funding; Xencor: Other: Serves as Principle Investigator. Tuscano: BMS, Seattle Genetics, Takeda, Acrotech, Genentech, Pharmacyclics, Abbvie: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal